Anti-mouse PrP antibody
Clone: MoPrp.03
Catalog No.: HR-Lympho-15
Host Species: Mouse
Reactivity: Mouse
Antigen/Immunogen: E.coli produced full lenght mouse cellular prion protein PrPC
Tested Applications: ELISA, WB
€200.00 – €1,500.00
Available Options:
Description
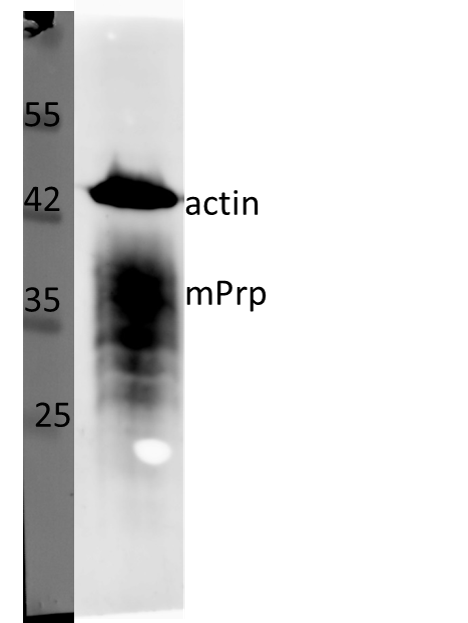
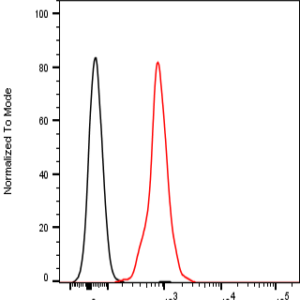
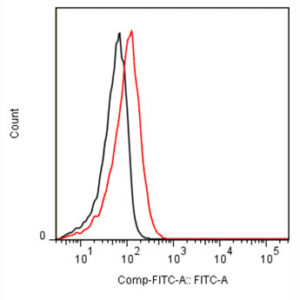
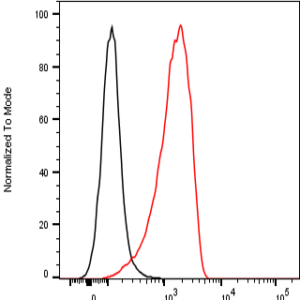
The monoclonal antibody MoPrp.03 recognizes mouse cellular prion protein (PrP), also known as PRNP.
PrP, is a ubiquitously expressed GPI-anchored cell surface glycoprotein associating with lipid raft components and functioning as a signaling molecule. The physiological function of the PrP is still poorly understood. It is involved in proliferation of epithelial cells and in distribution of junction-associated proteins in human enterocytes and it has been proposed to play a role in apoptosis and neuronal development. Recent findings suggest that it serves as a stress-inducible molecule and an immune cell modulator. Conversion of the normal cellular prion protein (PrPc) into an abnormal conformer (PrPSc) is the crucial step associated with triggering the pathogenesis of incurable neurodegenerative diseases, such as the Creutzfeld-Jakob disease (CJD). Our anti-PrP antibody MoPrp.03 has been validated in WB (cell lysate and immunogen) and ELISA (immunogen).
Additional information
| FORM | Liquid |
|---|---|
| STORAGE | Long term -20 °C, short term +4 °C. Avoid freeze-thaw cycles. |
| CONCENTRATION | 1 mg/mL |
| PURITY | Affinity purified |
| CLONALITY | Monoclonal |
| ISOTYPE | IgG1 |
| LIGHT CHAIN TYPE | kappa |
| REFERENCES |